Holograms on Wine Glasses and Glass Plates
This is the Blyth diffusion method for making silver halide holograms on glass plates and wine glasses.
 Jeff Blyth with horse hologram in wine glass
Jeff Blyth with horse hologram in wine glass
Contents
Introduction
This method is an advancement on my original system[i] where I first coated gelatin onto a glass surface and then diffused in silver salt to make it photosensitive. (This idea was then also found to be very useful to use on other pre-coated polymers).
Now a real effort has been made here to make this method useable for the home-based amateur using only items purchased from the Internet or from local shops. The new “age of terrorism” has now blocked the availability of chemicals from the large manufacturers to the private addresses of peace-loving DIYers .
The silver concentration used here is now three times higher than that used previously and this has raised the diffraction efficiency and much increased the photosensitivity. Previously it was found that pushing the silver concentration up to such a high level caused severe grain growth and grain growth has always been the bane of all silver halide holographic emulsion makers and even a century before them, for all emulsion makers for making Lippmann colour photographs. Here I have made 3 break-throughs in this new system. No.1: I discovered that I could greatly increase the concentration of silver ion without causing the dreaded severe grain growth phenomenon, provided that even the lowest levels of chloride ion residing in the pre-coated gelatin film could be first removed and that this could be simply done by first soaking the gelatin coating in a concentrated solution (soln) of potassium or sodium nitrate causing an ion exchange mechanism. No.2: I found that if I made my gelatin “emulsion” with silver chloride (AgCl) rather than the conventional silver bromide (AgBr), I was able to increase the photosensitivity by a factor of 3. The hologram still finishes up made in the conventional higher diffracting AgBr because I use a conventional bleach formula containing bromide ions. No.3: there is usually something present in shop-bought culinary gelatin which causes a degree of chemical fogging in the development step and that this could be prevented by simply adding a small quantity of copper sulphate to the gelatin soln prior to coating the film.
 By pre-swelling before the exposure is made with a HeNe red
laser, the holographic horse is yellow rather than red. By
preswelling still more the horse image is green as in above photo
By pre-swelling before the exposure is made with a HeNe red
laser, the holographic horse is yellow rather than red. By
preswelling still more the horse image is green as in above photo
Chemicals available from the Internet
The first 2 on this list are especially important to be the purest you can get off the Internet. The rest are less critical but should be better than 90% pure if possible. Some of these chemicals will be used for making up the developer and bleaching solution later after the plates are made. The chemistry there will be dealt with in a separate section.
Silver nitrate (AgNO3) This is available in 10g quantities. It needs to be as pure as possible via AMAZON or eBay.
Potassium Nitrate “Food Grade” via eBay or AMAZON. “Food Grade” has worked OK. But it does have the disadvantage of containing an anti-caking agent. When a 10% solution is initially made up, this agent causes haziness instead of making a completely clear solution (soln). This haze will also stain the finished gelatin film. So one must make up the soln well in advance before use, then allow the agent to settle out and then carefully decant off the upper clear soln into another bottle. High purity sodium nitrate would be best but most offers are of lower purity than the food grade potassium salt, the offers also are confused with the completely unsuitable sodium nitrite.
Sodium Chloride. Normal supermarket grade seems to be OK here. Even though it may contain small amounts of an anti-caking agent such as sodium ferrocyanide.
Chromium Potassium Sulphate (or Sulfate) via AMAZON or eBay. This should be a purple powder . It is also called Chrome Alum. The green powder sold as Chromium sulfate (basic) is not suitable. Straightforward “Alum” or aluminium potassium sulphate is apparently useless as a gelatin hardener for us here.
Acridine Orange via eBay (but not AMAZON). This is listed as “microbiological stain”. A bottle of 5g listed as “50%” from APC has worked well for use with a 532nm laser pointer .(Unfortunately the dye needed for red lasers I have only managed to get direct from Sigma-Aldrich or other big chemical companies , see section 19).
Methanol 1 litre via AMAZON or eBay.
Copper sulphate or sulfate. The blue crystals are easier to obtain than the white anhydrous compound, so my formulations are based on using the blue pentahydrate from eBay or AMAZON.
Developer chemicals.
Ascorbic acid (pure vitamin C powder). Ubiquitously available.
Metol From eBay
Sodium Carbonate anhydrous. From eBay, (this must not be the very impure “washing soda” crystals).
Sodium Hydroxide from eBay. A quite good grade is needed, (not the one sold just for clearing drains).
Bleach Chemicals
Copper sulphate (sulfate) (already listed above)
Acetic acid from eBay Potassium Bromide 200g analytical grade, available via AMAZON
Anti-Printout chemicals
(“Print-out” is an old term used by photographers. It means a darkening occurs in the finished photograph (or hologram) in sunshine or bright lighting. AgCl exhibits the effect more strongly than AgBr. Here holograms made from emulsions that originally contained AgCl rather than AgBr are more liable to print-out even though they finish up being made mostly of AgBr, they still retain some AgCl. So an anti print-out step is important.)
Sodium persulfate (or ammonium or potassium) from eBay.
Sodium hydrogen sulfate (sulphate) from eBay
Glass sheets. These are referred to here as “sheets” when buying the glass and “plates” when being turned into hologram recording material. It is convenient to use thin glass sheets of 2mm thickness for making small holograms up to say 5 x 4 inches for ease of cutting. However thicker glass is probably necessary for larger ambitious holograms because thin sheets can bow during exposure in a horizontal set-up.
I find it very convenient to use those 10 cm square disposable laboratory weigh boats (from the Internet) as dishes to hold the solutions needed to process an exposed hologram. The great feature of these boats is that when they contain about 60 ml (but not more) of developer, another boat can float on top and this can effectively keep the developer active for many hours whereas in an open uncovered dish oxygen absorption will exhaust the developer in about half an hour. Also although the other dishes needed are not all affected by oxygen, they can be affected by evaporation, so such lids are used on them too.
 Disposable 10 cm square weigh-boats used as trays to hold
developer and bleach.
A second tray acts as a floating lid to delay oxidation and
evaporation.
Disposable 10 cm square weigh-boats used as trays to hold
developer and bleach.
A second tray acts as a floating lid to delay oxidation and
evaporation.
To accommodate to these dishes I have been purchasing plates 2mm x 240mm x 160 mm. This is a convenient size for the gelatin coating procedure and is convenient for cutting into six 80 mm square plates that are a nice size for fingers to place in, and then take out of the dishes. Of course those fingers should be rubber-glove-covered. I also purchased 1 or more “carrier” sheets . These sheets were 4 mm x 270mm x 190mm. These thicker glass sheets allowed one to place the thinner glass on top with a 3 cm. border all round.
“Subbing” the glass. Failure of gelatin film to adhere reliably to the glass surface can be a major problem. A common way to get adhesion is to use certain silane compounds which cause attraction chemically to the silica constituent of ordinary glass. However I have tried 3 different brands of “silane” off the Internet which were sold as aids to help bonding when using epoxy resins. These were not fully satisfactory as they frequently failed to do the job. I found a better answer to this problem was as follows. I leave the glass sheets for half an hour or more in 100% domestic bleach. I then rinse them in running tap water while wiping them well with a cloth. I then mark the back of the 2 mm glass sheet with a black “permanent” marker pen with say a “B”. (If there happened to be a scratch on one side of the glass then that should be the side that will be gelatin coated, as the gelatin film will later hide the scratch). I then hold the sheet at an angle of about 45degrees and place a clean collecting tray at the bottom end. I then pour a very dilute solution of gelatin and chromium potassium sulphate (detailed below) all over the front of the tilted plate. Although quite dilute, the gelatin content in the soln is sufficient to reduce the surface tension enough to allow a film to form, rather than form droplets. This plate is then left to dry leaving a very thin film of gelatin and chromium salt. Once dry, the sheet was placed with the treated side face-up into a preheated oven at 190-200C for about half an hour. The gelatin soln for this subbing was a diluted form of the gelatin soln used to make the main coating soln detailed below. So 5 ml of the soln detailed below was added to 100 ml of warm water and then 5 ml of stock chromium potassium sulfate soln. was added. This stock chromium soln was 4% soln. of chromium potassium sulfate and 4% glycerol.
Stock Gelatin coating solution 1) I prepare a stock solution of shop-bought leaf gelatin of
12 g. in 100ml of cold deionized water. This is then placed
in a water bath to prevent the gelatin getting over-heated.
A thermometer is used in the inner gelatin bath to check that the temperature does not rise above 51 oC , while being constantly stirred for about 20 minutes or more. This is then filtered through a cotton handkerchief or nylon stocking to remove any froth from the surface About 0.5g copper sulphate CuSO4.5H2O is then added to about 3 ml deionized or distilled water, (DI) and the soln is then stirred into the gelatin soln. (There is often something in the shop bought gelatin that is inclined to cause mild chemical fogging. Also if the gelatin soln is over-heated this chemical fogging can become severe. This term “fogging “ is not related to the terms “haziness” or “scatter” mentioned later. “Chemical fogging” is a term used in photography to mean a degree of darkening (caused by fine silver metal grains) in developer not caused by light.
2) About 4ml of glycerol is then stirred in. I then pour it all into a clean bottle with a screw cap. Prior to coating I heat this stock solution up to 50 oC max. in a warmer. 3) I then take 2 glass sheets , one is 2mm x 240mm x 160 mm. (This size is convenient for cutting into six 80 mm square plates that will fit into those disposable 10 cm square weigh boats) . I use a 4mm thick glass sheet to act as a carrier sheet. The carrier sheet is 4 mm x 270mm x 190mm and the first glass sheet is placed on it, to make a margin of about 3 cm all the way round. Using PVC insulation tape, the thinner glass sheet is then taped down onto the carrier sheet with about 3mm of tape covering the perimeter of the thin sheet. I then place both this sheet combination and a clean plastic tray in the warmer together with the gelatin solution so that everything gets to 50 oC
 A 2mm thick glass sheet is taped onto a larger 4mm thick
"carrier" sheet.
A 2mm thick glass sheet is taped onto a larger 4mm thick
"carrier" sheet.
4) I then take out the warmed sheets and put them in the tray on a horizontal surface . Then working quickly I pour roughly 20 ml of the gelatin soln. onto the centre of the thin glass sheet and quickly spread the gelatin puddle all over the sheet by hand so that the whole area of the sheet is wetted by the gelatin soln. I then quickly lift up one end of the sheet pair leaving the other end in in the warmed plastic tray and incline the sheet at an angle of about 45 degrees and pour the rest of the gelatin across the top end so that it all runs down smoothly as I move the bottle across the top. I then lift the sheets up off the tray so that excess gelatin drips off the bottom for no more than 15 sec. (If the initial wetting with the gelatin solution had not been carried out then the poured film would not have properly covered the sheet evenly). 5) I then quickly place the sheets on a cool flat horizontally correct surface, free from strong air currents. 6) I then pour the remaining gelatin in the tray back into the bottle, screw on the lid lightly and put the bottle back in the oven at 50oC. for about 5 minutes before screwing on the lid tightly and storing the bottle at room temperature for re-use at a later date. (So far I have had no sign of any moulds growing after leaving the gelatin soln. at room temperature for 6 months like this). 7) When the gelatin film has gelled, I then remove most of its water content by leaving the sheet in a good flow of room temperature air from a fan. 8) When the gelatin film is touch dry I run a scalpel blade around the tape border to avoid any gelatin being torn off the glass when I remove the tape before the next step. 9) With the tape peeled off, and the gelatin touch dry I then place just the coated sheet in a clean tray, and pour over its surface a chilled soln (at~5oC) of previously made 4% chromium potassium sulfate with 4% glycerol. After about a minute, I remove the sheet and shake it, the sheet is very soft and vulnerable at this point, so I very gently touch any droplets still on the gelatin face with a tissue, and also wipe off the back of the sheet. I then stand the sheet up and leave it drying in front of a fan with a good air flow. Meanwhile I pour the chromium soln in the tray back into its storage bottle and keep it in a fridge for further use. (If the chromium soln later loses its purple colour and starts to look greenish then it should not be re-used). It is then left in a cold airflow overnight. (The gelatin hardening by the chromium salt only really takes place when the gelatin film is in a relatively dry and unswollen state. An oven or warmer at 50 oC could be used instead to speed the slow process up to about an hour, but the gelatin film MUST firstly be made dry under a cold blower before being put in a warmer). If the room is below 20 oC it is best to use the warmer anyway, otherwise the slow hardening process may still be incomplete after 12 hours. 10) I then rinse the sheet front and back in running tap water and then with 3 changes of DI water. (Unlike the notorious dichromate which is (CrVI), it is OK here to let a small amount of this form of chromium (CrIII) go down the drain). 11) The sheets are then left in a bath of 0.10% sodium carbonate for about 2 minutes only and then briefly rinsed in DI. (Making the gelatin slightly alkaline contributes greatly to its ability to bind silver ions later). 12) The sheets are then left for several hours or overnight in a 10% soln. of pure sodium or potassium nitrate for the essential process of removing any chloride ions by a slow ion exchange mechanism. This time can be cut down to about an hour if the solution can be continuously mechanically agitated. The soln can be reused a number of times, depending on the amount of Cl ion it has exchanged for NO3 ion. Note that if the nitrate is from “food grade” potassium nitrate it should be a clear solution that has been decanted off from a whitish precipitate of anti-caking agent that has been allowed to settle out from an initially hazy solution. 13) The sheet is then rinsed well in DI and dried in a cold air flow so that it will be ready to allow silver nitrate to diffuse in. N.B. I have to be very careful to avoid any contamination from traces of chloride ions on the wet gelatin at this point. e.g. from tap water splashes, fingers, sneezing over it or forcefully pronouncing words with the letter “P”!!! (Otherwise tiny amounts of AgCl produced from stray Cl ions will grow into horrible big white grains in step 20 later.) 14) A concentrated stock soln. of silver nitrate (AgNO3) is prepared by making a solution of 10g of silver nitrate crystals to 50 ml of DI water in an ultra-clean bottle with lid. (This relatively expensive solution is very vulnerable to contamination. As well as any chloride or bromide traces, I am very careful not to allow any paper tissue particles to get into the stock bottle as such organic stuff can cause the soln to go brown due to colloidal silver formation, which can cause bad fogging during the development of the plates. ). 15) I then use the thick glass carrier sheet (carefully cleaned and free from halide traces) as a flat surface to coat the gelatin film with the silver nitrate solution by a puddle method . The room lighting does not need to be at a low traditional darkroom “safelight” level at this point. A low wattage tungsten filament bulb (eg. around 15W) is OK but not fluorescent “daylight” lighting. Using a clean syringe or pipette, I deposit a certain sized blob of silver nitrate soln. close to an edge of the carrier sheet. I then carefully place a cut piece of the gelatin coated sheet on top of the blob so that about a half to one cm. of the sheet protrudes over the edge of the carrier glass. The blob of silver nitrate soln then spreads evenly and easily over the gelatin film by capillary action except of course for the bit protruding over the edge of the thick carrier sheet. This overhang bit is useful for handling the sheet without touching the silver nitrate. The AgNO3 is allowed to soak in for about 2 minutes only. The size of the blob is roughly as follows; for an 8 cm square plate, my blob is made from about 0.8ml of the Ag solution. (Remembering that each 1ml blob will perhaps have cost you nearly a dollar in 2016). The puddle coating system usually works particularly well if the carrier sheet is first treated with “Rain-X” which is obtainable from suppliers of automobile sundries. This stuff makes the carrier glass water-repellent and helps to restrict the silver nitrate solution from straying beyond the thin glass sheet boundary). 16) The gelatin film then needs to have any AgNO3 soln. on the surface to be wiped off with care. A small car windshield wiper blade can be used as a squeegee and the rubber washed clean in DI after each use. Alternatively a clean doubly folded tissue folded over a ruler can be used as the wiper blade but using only one quick single wipe per tissue. (The tissue may contain low amounts of Cl- which could flow into the gelatin if more than a quick single wipe is used). 17) The plate is finally dried in a cool air flow, I check to see that there is no scatter at this point. If there is just the slightest mild scatter over the plate at this point then the plate should be discarded, (unless it was just caused by that anti-caking agent in KNO3). Because it means there was still some chloride ion contamination in there and the scatter will get much amplified in the step using a chloride and dye bath in section 20. If there is scatter in just a small part of the plate area then that probably means some chloride or bromide contamination from handling fingers that had been in tap-water, but it may be worth continuing by ignoring a small scattery area around the edge.
Preparation of stock sodium chloride and dye solutions.
18) I prepare a 1 litre soln as follows. I add 41g. sodium chloride (NaCl) to a 1 litre bottle and add 334 ml of water, then after it has dissolved, I pour in 666 ml of ethanol or methanol. This makes a 2:1 alcohol : water soln. (These quantities do not have to be that precise, even a 10% error will probably be OK). 19) For the 8cm square plates, I take a 100ml of the stock NaCl soln. and add 2 ml of stock dye soln , then I use about 70 ml of this soln in one of those 10cm square dishes . The stock dye solutions are made as follows. For green lasers I prepare 0.5g of Acridine Orange (A.O.) per 100 ml of a 2:1 ratio of alcohol :water. The A.O. I got off the Internet is described as “50%” acridine orange.—It has worked well. For red lasers I use pinacyanol chloride, (its old name was “quinaldine blue” ). I prepare a stock soln of 0.3g of pinacyanol chloride in 100ml ethanol or methanol, (pinacyanol bromide or iodide can be used instead). It is most regrettable that I have not managed to buy this dye on the Internet as an ordinary citizen yet. This is the one item that I have had trouble with. A Chinese company does offer pinacyanol iodide on the Internet but the shipping costs quoted for just 1 gram are utterly ridiculous. So my dye had to be bought professionally from Sigma-Aldrich). 20) So to make my plate photosensitive I now have to work under an appropriate safelight; dim green for making red sensitive plates and dim red for making green sensitive plates. The safelight does not need to be as dim as was used for traditional photographic darkrooms where much more photosensitive material was used. One can get an idea of how much light of the wrong colour is coming out from your safelight from looking at the rainbow spectrum you get off the surface of any DVD held in the area of the room where you are working. I find that the “REMOTE CONTROLLED AUROGLOW” bulbs obtainable via AMAZON are excellent as safelights as they have instantly selectable colour and brightness . 21) I take the dried plate containing the AgNO3 and plunge it quickly into the chloride/dye bath for 45 seconds , agitating it constantly. (The time in this bath governs the grain growth. If it is too long the grain growth can get unacceptable, whereas if it is too short then a lot of silver nitrate could remain unconverted to silver chloride). After the 45 seconds I then without delay plunge the plate into a dish under cold running tap water and I leave it in for at least a couple of minutes or more. 22) Then the plate is inspected under reflected safelight. It must look scatter free or at least only show a very slight haziness . If there is bad scatter then the probable cause is a failure to remove traces of chloride ion effectively from the gelatin before the silver nitrate soln was applied. If the scatter is slight then it may be worth continuing anyway. Scatter just around some of the edges is quite likely to occur due to increased thickness and handling. The edges should not be included in the final holographic image anyway. Accidental areas of much greater thickness may also still contain unconverted silver nitrate. Such areas will turn rapidly black due to a deposit of silver metal when they meet the sensitizing bath of 2% ascorbic acid discussed in the next paragraph. They would also fog badly in the final development step of course. If one tries to eliminate any unconverted silver nitrate in accidentally thick areas around the edges by prolonging the time spent in the halide/dye bath, then an increased level of grain growth occurs in the thinner areas that are OK after just 45sec. of immersion only. The best one can do if unconverted AgNO3 is still thought to be lingering in thickly coated areas is to prolong the time in the running tap water, e.g. for 10 minutes, (the water should be as cold as possible). To maintain a final replay colour close to that of the laser wavelength, I then put the plate in a bath of 2% ascorbic acid (vitamin C) that has been taken to a pH of between 5 and 6 with sodium hydroxide. (If you have not got pH paper available then carefully weigh out 2.0 g vitamin C and dissolve it in 100 ml of DI. Then add 0.38g sodium hydroxide). Without this vitamin C treatment the light sensitivity of the plate is very low. I then wipe off the droplets with a tissue or give the plate the briefest rinse in DI. (I like to leave a trace of ascorbate in the plate as it helps to maintain its photosensitivity). 23) If I want to make bright green holograms from a red laser, then I must not use the ascorbate bath but instead I immerse the plate in a bath of 15% triethanolamine or ”TEA” for about a minute. (Treating with both ascorbate and TEA causes chemical fogging or darkening without light involvement). 24) I then carefully wipe the surface free of droplets using either a wiper-blade squeegee or doubly folded tissue over a ruler similar to that used for the earlier AgNO3 treatment, (but now I do not have to worry about any contamination from chloride ions). 25) I then leave the plate under a cold air blower for 20 minutes to acclimatize with ambient humidity. It is then best to leave the plate in a cardboard dark box until the next day when you are making larger holograms because the gelatin surface is probably not going to be stable enough for longer exposure times of more than say 4 seconds. However you will probably want to at least make some necessary exposure tests on small plate pieces before that. I find it best to do initial tests on a glass top table with the spread laser beam coming up from underneath the table or any frame able to hold a horizontal glass sheet. Initially it is best just to make a photographic exposure test. With the beam shuttered I place a piece of my recording plate on top of some masked off area , such as the piece of negative with lettering on it in the image below hat had been put in the beam before it was shuttered. The laser exposure times need to be sufficient to give a development time of about half a minute or less at 22C (see below under “Development Time” discussion). The “photographed” unmasked off area should look quite dark whereas the masked off area should be no more than slightly dark, ( if it is nearly as dark as the unmasked area than that means that I have a fog problem either caused by stray light or chemistry in the gelatin).
 This is the rig I use for initially testing newly made
hologram recording plate. A sheet of 5mm thick glass rests
over a frame of a stool. Under the glass sheet I have a 4 mw
red laser pointer sitting in a glass jar. The laser has been
treated as per the images below. The beam is shuttered off
with a piece of black card stuck to an upturned plastic
beaker. On top of the glass are various objects for making
exposure tests. I often start with making just a
photographic image of a piece of film negative with
lettering by placing the lettering in the centre of the beam
and then with the beam shuttered I put a piece of plate
ontop of the negative for an exposure test. To make
holographic tests I place objects ontop of a piece of plate
and give them time to settle before exposing. The aluminium
cups are useful for putting over objects so that more laser
light can be used to illuminate the sides of an object
provided the laser has enough coherence length (see final
section "Lasers"). This simple set-up is very good for
making simple holograms in unstable environments.
This is the rig I use for initially testing newly made
hologram recording plate. A sheet of 5mm thick glass rests
over a frame of a stool. Under the glass sheet I have a 4 mw
red laser pointer sitting in a glass jar. The laser has been
treated as per the images below. The beam is shuttered off
with a piece of black card stuck to an upturned plastic
beaker. On top of the glass are various objects for making
exposure tests. I often start with making just a
photographic image of a piece of film negative with
lettering by placing the lettering in the centre of the beam
and then with the beam shuttered I put a piece of plate
ontop of the negative for an exposure test. To make
holographic tests I place objects ontop of a piece of plate
and give them time to settle before exposing. The aluminium
cups are useful for putting over objects so that more laser
light can be used to illuminate the sides of an object
provided the laser has enough coherence length (see final
section "Lasers"). This simple set-up is very good for
making simple holograms in unstable environments.
For a holographic test, the beam needs to be almost but not exactly perpendicular to the plate to make the traditional test with polished coins. With the beam shuttered, the coins are placed on top of the recording plate preferably on the glass side of the plate, and left for at least 10 minutes to settle. A newly made gelatin film may contract by a nanometre or two during exposure while it is thus sandwiched between the 2 glass sheets but simple coin holograms can still be obtainable because the coin to “emulsion” distance should stay the same at least for most of the emulsion attached to the plate’s glass. The important point about this test is to see how quickly the exposed hologram takes to darken in a developer such as TJ1 (see below). This glass table top system is excellent for making holograms in an unstable environment because the plate and object can “ride the storm” together.
Coating Curved glass surfaces such as wine glasses.
 Martini glass with cut-off stem to afford possibility of constrcting a hologram with an all-round view
Martini glass with cut-off stem to afford possibility of constrcting a hologram with an all-round view
Wine-glasses have the very convenient property of being able to act as their own processing baths. It is best to do several glasses at the same time. I recommend getting 2 packs of 4 cheap plain wine glasses of about 250 ml (cc.) capacity.
I first fill the glasses to the rim with either undiluted or 50% diluted domestic bleach solution. The bleach is left in each glass for about 15 minutes or longer. The bleach is then poured back into a bottle for re-use, and the glasses are rinsed well in running tap water and wiped around with a cloth before being given a rinse in DI.
Each glass is then coated with the subbing layer described above under the title “Subbing the glass” but this time more conveniently, the subbing solution is poured into the glass and the glass is then tilted so that the soln wets the side of the glass up to the rim and the glass is then rotated so that the whole glass is wetted, the soln is then poured into the next warmed glass and so on. The treated glasses are then left standing in an airflow so that the very thin subbing film gets dry. The glasses are then placed in a preheated oven at 190-200C for about a half-hour. They are then allowed to cool and are given a rinse in DI. The glasses are then placed in a warmer at 50 C to dry. The bottle of stock gelatin solution should also be in the warmer and also a plastic or glass beaker with a spout.
A typical wine glass holds about 250 ml. of soln. So I take 100 ml (or it can be less) of the 50 oC gelatin stock solution prepared as previously described and I pour it carefully and slowly into the warm beaker while trying to avoid creating any bubbles. The beaker is then used to carefully pour the gelatin soln into the wine glass without creating bubbles, (the spout makes this requirement easier than pouring straight from the warm stock bottle). The wineglass is then tilted and rotated so that the inside is all wetted by the soln up to the rim of the glass. The gelatin is then poured slowly from the glass back into the beaker trying to again avoid creating bubbles. The glass is then upturned and placed on a clean surface allowing the gelatin solution to flow down to the rim before gelling at room temperature. Meanwhile the beaker of gelatin soln is poured into the next glass at 50 oC and so on. The soln in the beaker should not be allowed to drop below say 40 oC or it may get too viscous to coat properly. (I do not recommend using a few seconds in a microwave oven to reheat it as I find that it can damage the gelatin and cause fogging later).
Once the glass is cold and the gelatin film has gelled, then just the rim of each glass is carefully dipped into a bath of warm water to remove that thick gelatin layer around the rim. I try to not let this water level go higher than about 3 mm up from the rim. The glass is then still held in an upturned position and just the rim is wiped free of water droplets so that these droplets will not run down into the glass when it is placed upright. The upright glasses are then placed in a strong cool air current to remove the excess water in the gelatin. Once the gelatin film is unswollen (except for the partial swelling caused by the glycerol present), it is ready for the hardening solution to be poured in. The hardening soln is as described previously. Namely 4% chromium potassium sulfate soln with 4% glycerol chilled to about 5 oC. This solution is swirled around the wineglass so that it wets all the gelatin film and is then emptied into the next wineglass and so on. If the chromium soln has not run down the sides of the glass evenly when the glass is stood upright then any droplets should be gently removed by just touching with a paper tissue (do not wipe as the gelatin is very soft at this point), as only the absorbed chromium solution is wanted and droplets can cause a mark on the surface later. The wineglasses are then left upright in a cool airflow for several hours to harden. This hardening process is slow and if the room is particularly cold overnight say then it is best in the morning to warm the glass in a warmer at 50 oC for 20 minutes or more, or with a hairdryer at about 50 oC for a few minutes to finish off the hardening process.
Any excess chromium salt is then removed under cold running tap water. [Unlike the notorious dichromate which is (CrVI), it is OK to let a small amount of this form of chromium (CrIII) go down the drain]. The glass is then shaken free of tap water droplets and rinsed at least three times with DI.
Each glass in turn is then filled to the brim with a 0.1% solution of sodium carbonate for about 1 to 2 minutes and is then rinsed briefly in DI.
Now follows the vital step of removing traces of chloride ions from the gelatin film. Each glass is filled to the brim with about a 10% solution of potassium or sodium nitrate. They are then left for several hours for the ion exchange process to occur. (Cl exchanged for NO3). The nitrate solution is then poured back into a bottle where it is probably reusable several times more. The glasses are then rinsed in DI three times and left in a warm air-flow. When dry they are ready for loading up with the silver soln.
Diffusing in silver nitrate solution
For your first wine-glass experiments it is best to only expose one side of the wine glass and therefore you should only put your expensive silver solution on one side of the glass. Covering the whole glass with silver causes real complications for an object such as a model figurine, because it will record a photographic shadow on the opposite side of the glass. It will also record spurious rainbow coloured effects due to transmission type gratings. However, it is worth covering the whole glass with silver solution if you want to just do a simple hologram of the glass filled to the brim with say coins. Then 2 exposures can be made 180 degrees apart and each exposure will not be affected by an exposure on the opposite side of the glass.
So for making a reflexion hologram on one side of the glass only, you need to first decide which half of the coating looks the best to use and then use a marker pen to put a cross on the other side that you do not want to use. To put the silver solution into one half of the gelatin film you need a new disposable rubber glove at least on one hand. Using a 1 ml clean plastic syringe take 0.5 ml of the silver nitrate solution, and holding the glass horizontally with the future image side down, gently empty the syringe onto that concave surface. Then use your gloved forefinger to wipe the silver solution only over that designated half of the glass. Spend at least 2 minutes gently wiping that solution over just that half of the glass. At first it might seem that the solution is not wetting the film surface well and is forming droplets. However as you work at rubbing it into the gelatin, the gelatin’s surfactant properties start to kick in and the silver soln spreads more easily. This operation can be done in subdued ambient lighting such as that from a 25W or 15W tungsten filament bulb, there is no need for a proper safelight at this stage but it is vulnerable to fluorescent or blue-rich lighting. Once the gelatin layer has absorbed the silver solution, it is important to remove any excess solution off the surface or it will scar the finished hologram. I use a folded tissue to gently wipe the gelatin surface free of solution. Only use the tissue for one single quick wipe then throw it away and use another clean tissue if needed. If you plan to use the whole glass for 2 images on opposite sides then you will need to use 1.0 ml of silver nitrate soln and spend at least 3 minutes rubbing the solution over the whole surface before wiping off the excess with tissues.
The glass should now look clear and scatter-free at this point, a hairdryer is now needed to dry it in a tepid airflow because it must be fairly dry for the next step, but a hot blow must not be used.
Sensitizing the gelatin film.
Suitable safe lighting is now needed for the next steps.
The average wine-glass will take 250 ml of liquid. So it can be convenient to pour say 300 ml of the stock sodium chloride (section 18 above) into a beaker and add about 6ml of the stock dye solution (section 19). Then about 250 ml of it can be rapidly poured into the wineglass and left in for only 45 seconds and then quickly poured back into the beaker and the glass then put into running tap water with minimal delay. A beaker should be used each time for each glass rather than pouring from one glass to the next. The timing here is important because if it is too short , not all the silver nitrate in the gelatin will be able to be converted to silver chloride and if it is too long there will be quite rapid grain growth. The thickness of the gelatin layer is also a factor in determining the optimum time. To stop the reaction of the sodium chloride solution, the glass is filled rapidly with running tap water and left in the water for several minutes. It is then shaken free of tap water and given a brief rinse in de-ionized water and then filled with about 3% vitamin C solution that has been taken to a pH of between 4.5 and 5 with sodium hydroxide or with sodium carbonate (a pH of over 7 acts as a developer and will cause chemical fogging) the glass is then given a brief DI rinse and left to dry in a good cool air flow. If you wish to use a red laser to make bright yellow-green holograms then the ascorbic acid treatment should not be used and should be substituted with a soln of 12% TEA. After a minute or two in this TEA solution, the gelatin surface should be gently wiped with tissues so that no droplets or rivulets remain. The outside of the glass should be wiped too of course so that it is smear free. The photosensitive glasses should then be dried in a good air flow and left to equilibrate in a light-tight cardboard box or cupboard. They should not be used till at least a day has passed because the gelatin layer will probably be quite unstable for many hours.
The Developer
It is most convenient to have a 2 part developer in two one litre bottles labelled A and B. The developer can keep indefinitely in these separate bottles. They are then used by mixing equal volumes.
The developer below has been christened “TJ1” developer. (In honor of the late Tung Jeong who asked me to make a suitable developer some years ago for teaching his students).
Part A
- 6g Metol (4-methylaminophenol sulfate)
- 1 litre deionized water
Dissolve up first then add:
- 40g. Ascorbic acid (vitamin "C")
(Without the Metol, the developer will still work but is slower to act and the results may be less good.)
Part B
- 100g sodium carbonate anhydrous
- 30g sodium hydroxide
- 1 litre deionized water.
(This one should be labelled "very caustic" use rubber gloves and eye protection --guard against splashing it around.)
Just use equal volumes of A and B. For the 8 cm square flat glass plates, use the "floating dish" method. Two close-fitting plastic dishes are arranged so that one floats on top of the other. The volume in the lower dish should be just enough (50-60 ml) to give a minimal air gap so that the uptake of oxygen is minimised and the top dish can be used as a rocker to agitate developer over a plate.
In the case of wine glasses, they can be rapidly filled to the brim with developer but because the development time needed may be only a fraction of a minute, I find it best to first wet the exposed glass with DI and then rapidly pour in roughly 60 ml of developer and then twirl the glass around while looking through it at the safelight with one eye closed to judge when the amount of darkening is sufficient as described in the next paragraph. The development does not stop by emptying the developer out into a beaker and will continue on while you are inspecting the darkening. It is stopped by giving the briefest rinse under running tap water and then pouring in the bleach solution.
Development Time
Developer TJ1 is intended to react quickly (to keep the silver grains spheroidal rather than filamentary, and to minimize damage to the gelatin in the strongly alkaline solution). So sufficient exposure level to give a development time of only 15-30 seconds should be aimed for. To judge when the time is enough if you have not had previous experience is a little tricky because it is rather subjective. Here is my method for beginners:-
Take a small sample of the holographic recording material you will be using and totally expose it to bright daylight for at least 5 minutes. Then put it in developer for a minute. Then wash it well in a bowl of tap water and dry it . Now keep that piece of plate or film as a reference for your dark room. With your eyes adapted to your darkroom safelight, shut one eye and look through that piece of reference plate at your safelight.( Holographic recording material is never completely opaque). The degree of transparency or optical density of your reference plate tells you that the darkest parts of your developing hologram must never get as dark as that reference plate as judged with one eye shut. I just go for a “half as dark” subjective estimate, while holding the developing plate up to see the safelight through it. I then plunge plate into a dish under running tap water and then into a “stop bath” of 5% acetic acid, or straight into a dish of bleach solution which also contains a lot of acetic acid and oxidant which rapidly stops the development process in the plate. Ordinary room lighting can then be switched on.
It is best to first wet your plate in DI before putting it in developer because it helps to even out the development over the plate area when the development is going to be rapid. If the plate in the white dish of developer appears to go black in only about 5 seconds then that means the development should be brought to a halt within about 15 seconds (allowing for that very rapid one eye inspection of the safelight). In that case do not carry on developing for a minute just because that is a more conventional developer time. More often than not, a rapid development and rapid stop in a bleach bath give the brightest results. However, if the film only starts to darken after about 15 seconds then about a 1 minute development time is probably about right in TJ1 developer but it may still need to be longer. Ideally the laser exposure times need to be sufficient to give a development time of not more than half a minute at 22C . A room temperature below 20C will however slow the whole process down. The development is stopped by first rapidly plunging plate into a big tap water bath for a second and then into a bath of the recommended bleach soln.
The developer's useful lifetime with the floating dish method can be days, depending on usage. A yellow or mild brown colour means the developer is still good. When the developer is very dark brown it should be discarded. (It is the Metol constituent that is causing the brown colour when it is oxidised and it acts as a helpful indicator of exhaustion).
Bleaching solution
For reflection holograms, I find the most easily obtainable bleach is made as follows:-
- 20g. Copper sulphate (CuSO4. 5H2O)
- 80g. Potassium bromide (or sodium bromide)
- 70 ml Acetic acid
- DI to 1 litre.
This is known as a rehalogenating bleach. (This is not the best for transmission holograms however). The bleach does promote grain growth, so the time in the bleach should be minimized. The bleach process should not be allowed to take much longer than about a minute. If there is a very dark area (due to over-developed up silver) that has not gone after about a minute and a half , it is best to stop the bleaching reaction anyway and wash the plate under running tap water . That still dark part will clear in the final bath used next. As this bath does not contain a lot of bromide ions there is no tendency for it to encourage more grain growth.
After rinsing well under tap water, a final bath to prevent “Print Out” is needed.
Anti-Printout stock solution
“Print-out” is an old term used in photography. It means a darkening occurs in the finished photograph (hologram) in ambient lighting , particularly in sunshine.
- 40 g. Sodium persulfate (or ammonium or potassium persulfate)
- 40g. Sodium hydrogen sulfate
- DI to 1 litre.
3 minutes in this bath followed by a very brief rinse in DI gives good print-out resistance. (Always make sure that your final rinse water is free from any traces of developer ).
Bleach for transmission holograms.
The anti-printout solution above will also work as a bleach for transmission holograms. This type of bleach is known as a “solvent bleach” which means that all the developed-up silver goes into solution and the final hologram will be made only from the original silver halide in the dark fringes that did not develop up, leaving the light fringes as just gelatin. It is very important in this type of bleach to have the developed-up plate well washed in DI before going into the bleach bath. This is because the plate has to be free of soluble bromide salt as does the bath itself. Then after the bleaching process is complete the plate should again first be rinsed in DI water before being given a final tap water rinse. The reason for this requirement is as follows. A final hologram’s diffractive efficiency relies on the refractive index difference between its light and dark fringes i.e. its fringe contrast. In the solvent bleach system the fringe contrast is got from silver halide left in the dark fringes against just gelatin in the now emptied-out light fringes. The removed dark silver metal is now ideally all in solution as soluble silver sulfate. However if any soluble halide ions were still present in the gelatin then these will combine at once with the developed-up silver as it is being oxidized to silver sulfate and the silver will be deposited back in the light fringes as silver halide where it will spoil the fringe contrast because the light fringes will not be just emptied out gelatin. In case you are wondering where soluble halide ions in the gelatin have come from, the answer is that they are always going to be produced by the developer---as the silver bromide gets converted to dark silver metal and the bromide goes into solution as sodium bromide salt.
Lasers
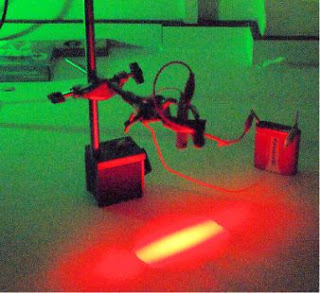
 The set-up for exposing the horse hologram is shown here
under HeNe light. The horse is suspended half way down the
wine glass by means of a screw that passes through a metal
bar which lies across the rim of the glass where it is fixed
with glue-gun adhesive. The screw is made invisible by
wrapping it with black flock paper.
The set-up for exposing the horse hologram is shown here
under HeNe light. The horse is suspended half way down the
wine glass by means of a screw that passes through a metal
bar which lies across the rim of the glass where it is fixed
with glue-gun adhesive. The screw is made invisible by
wrapping it with black flock paper.
Ideally one needs to use a laser with a good coherence length. What this means in simple terms for making holograms using one beam is as follows. The diverged laser beam has to pass through the recording plate and illuminate a stable object on the other side of the plate, and then the light from the object has to pass back through the plate and form standing waves with the incoming light. These are recorded as “fringes” running inside the gelatin like the pages of a book. This forms a reflection hologram also known as a Denisyuk hologram. If the laser light were made of just one single wavelength that would be perfect because the standing wave pattern would be continuous from the plate to the object regardless of how far away the object was, provided that enough light comes back from it to form recordable light and dark fringes. However lasers are rarely so perfect and the light is usually made of a very narrow spread of wavelengths. If the object is too far back then the returning light gets out of step with the incoming light and instead of the crests and troughs of the waves coinciding they clash and fail to form the standing wave pattern. (But, at a still greater distance they do come back into step again). From the earliest days of holography, the main workhorse laser was the Helium-Neon or HeNe laser. This can give a depth of over 20 cm. in a hologram using the red 632.8 nm wavelength. However in recent years compared to the cost of a HeNe laser, incredibly cheap red laser pointers have become available and I have found that in the case of the cheapest small ones (~5 mw) I can get a surprising depth of several cm. operating in the correct mode. (A wrong mode causes striped patterns in the hologram but this effect can be temporary). The much more powerful red laser pointers with powers around 50 and 100mw., I found quite useless for holography unfortunately.
This shows the simple operation of hack-sawing off the lens of a small red laser pointer (~5mw) to give a very nice bar of clean light. For stability, the 3 button batteries needed to be replaced by the equivalent 4.5 volts from a large battery with the help of wired up and partially insulated croc clips. These little laser pointers operate at a much lower current than is the case for the cheap green laser pointers and therefore temperature stability is not much of a problem.
With a cheap 532nm green laser pointer , I have managed to get a hologram depth of just over 3 cm once it had achieved temperature stability after a 10 minute warm-up period using the type shown below. This time after unscrewing all detachable bits and then sawing the lens off the end, I got a roughly circular spread beam, part of the other end of the barrel is also sawn off so that I could attach a variable 3 volt d.c. power supply using croc clips. It is a good idea to hold the laser in a lab clamp and stand with only bare metal on the clamps jaws (not cork or rubber) so that it will help conduct the heat away and reach an equilibrium temperature for stability. Green lasers in the photo seem to operate with a current around 0.6 amp. But if they get too hot the light output can suddenly drop very low. The original battery supplied was listed as 3.7 volts and so this value must not be exceeded and it is best to work below this voltage level. If a holographic image shows a weird stripey image, that means the laser was operating in an unfavourable mode.
Slightly changing the voltage can often make the mode O.K. but I am afraid one can be unlucky with these very cheap lasers. I am very pleased with the luck I have had so far with such cheap laser power. With the cheapest narrower pen-like green laser-pointers, operating with two AAA batteries I found I could only get a hologram depth of little more than 1 cm, but this was still enough for making gelatin holograms of coins or of slightly angled flat mirrors to make useful sensors for moisture[ii] or protease enzymes[iii]. However, for serious holographic imaging one must spend more serious money getting a laser with a guarantee that it will operate in what is known as TEM00 mode and a coherence length of over 20 cm.
[i] Blyth J. et alia The Imaging Science Journal Vol 47. 87-91 (1999)
[ii] Holographic Sensor for Water in Solvents
Jeff Blyth · Roger B. Millington · Andrew G. Mayes · Emma R. Frears · Christopher R. Lowe Apr 1996 · Analytical Chemistry vol 68, 1089-1094
[iii] A Holographic Sensor for Proteases
Roger B. Millington · Andrew G. Mayes · Jeff. Blyth · Christopher R. Lowe Dec 1995 · Analytical Chemistry vol 67 4229-4233