Dichromated Gelatin
Gelatin is the common base for photographic film and plates, including holographic materials. Polyester film or glass plates are coated with a thin layer of gelatin infused with silver halide and other chemicals that give the film its light-sensitive properties. The gelatin is the vehicle to carry the silver halide in place during film storage, exposure, and development. Otherwise, it is inert.
However, when ammonium or potassium dichromate is mixed with the gelatin, the gelatin becomes weakly light-sensitive. This opens a door to another arena for the holographer, entirely different from the more traditional silver-halide process. The grossly simplified explanation is that light in the presence of dichromate molecules cause hardening of the gelatin. The hardened gelatin diffracts light differently from unhardened gelatin, so the gelatin can record a diffraction pattern necessary for a hologram. An exposed dichromated gelatin plate is "developed" by first removing the dichromate in a water rinse, then removing the water through a sequence of alcohol baths.
Contents
Basics
Dichromated gelatin, or DCG, holograms are phenomenally bright. Unfortunately, gelatin is hygroscopic, meaning it has a tendancy to absorb water, and as the gelatin reabsorbs the water removed by the alcohol baths, the hologram will fade away. To preserve the hologram, it must be sealed from moisture, usually with some type of epoxy.
Dichromated gelatin is roughly 1,000 times less sensitive to light than the typical silver halide film, and even then it is most sensitive at the short end of the light spectrum (blues and violets), moderately sensitive to greens, and virtually blind to reds without some complicated chemical treatment to improve its red sensitivity. Most DCG holograms are produced using green or blue lasers at the higher end of the power scale, 100 mW or more at 532 nm.
A serviceable 100 mW green laser suitable for holography may be out of reach of the hobbyist wanting to just give DCG a try. Nonetheless, modest sized holograms can be produced with modest power lasers. The image shown here is of a hologram created by a newcomer to DCG techniques, John Fisher. A handful of 1/4"-20 socket-head screws were the subject; the glass plates, 2 x 3" large microscope slides, and the laser used was a Coherent C215M-10 operating at a lowly 10 mW.
Overview of the Process
Conceptually, making dichromated gelatin plates then processing them after exposure is fundamentally simple. The emulsion is made from ammonium or potassium dichromate, gelatin, and water. Often the formula used is expressed as a series of three numbers, where the numbers represent the ratio of the three components. The recipe, 5-30-250, means 5 grams of dichromate to 30 grams of gelatin to 250 milliliters of water (also grams). The gelatin is first added to cold water and allowed to swell, the mixture is heated while stirred continually until the gelatin is completely dissolved. The dichromate is then added with continued stirring until completely dissolved.
The mixture of water, gelatin, and dichromate, still warm, is applied to glass plates. The simplest method is called veil coating in which the mixture is poured onto an angled plate and allowed to flow over it. Other methods include first pouring a line of mixture at one end of a plate then using a Meyer bar or doctor's blade to drag the mixture across the plate. Spin coating is possible, but at much lower rotational speeds then is used for, say, integrated circuit production, and tends to be very wasteful of mixture. Low-speed spinning can be used after any of the other coating methods to get a more even distribution.
Coated plates are then allowed to dry. They are usually ready for exposure 4 to 12 hours later. Since dichromated gelatin can be 1,000 times less sensitive to light at 532 nm (for example) than silver halide emulsions, exposures may take a while.
After exposure, the plate is left to sit in the dark for a few minutes before processing begins. Fixing is first, and this can be done either chemically with a standard photographic fixer or optically with a few seconds exposure under intense white light. Washing is next to remove the dichromate from the gelatin. Finally comes the drying step, and this involves a sequence of progressively more concentrated isopropyl alcohol baths, the last bath being 99% alcohol. Hot air drying is next to remove the alcohol.
Once completely dry, the DCG hologram can be quite spectacular. The diffraction efficiency can be over 90% with no noticeable grain, and the hologram itself is very bright. Unfortunately, it will not last if the hologram is not sealed. Gelatin is hydroscopic, and therefore absorbs water. As water returns to the gelatin matrix, the hologram disappears. A common method to seal the hologram is to epoxy a second glass plate to the back of the hologram plate, thereby protecting it from moisture.
More Information
- A Beginner's Approach to DCG Holography has a more detailed and complete description of the whole process.
- A Simple DCG Recipe delivers a simple, just-do-this explanation.
- Dichromated Gelatin Chemistry has pointers to several DCG-related topics.
The Mechanics of Gelatin in the Dichromated Holography Process
By John Pecora
There is a lot of information available on collagen, gelatin and dichromated gelatin (DCG) holography but a paper that ties together these facets and can be understood by the amateur holographer is simply hard if not impossible to find. The scope of this paper is to finally bring together a concise understanding of what is happening in the DCG process. As it is impossible to footnote exact portions studied from other works because I intend to combine all research, I will simply put the credit due to the works I studied at the bottom of this paper and leave it up to the reader to research the individual papers for verification of the information I found.
Collagen
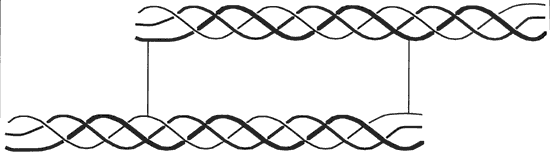
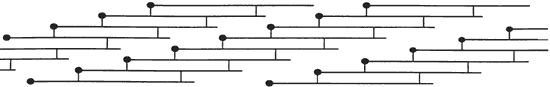
Collagen is a protein found in the skin, bones, tendons, cartilage, teeth, ligaments and connective tissue. It is the supporting structure for most body tissue. The collagen molecule is about 300 nm long and 1.5 nm in diameter. It is made up of three polypeptide strands, each of which is a left handed helix. These three left handed helices are wound together into a right handed triple helix. The strands are stabilized by hydrogen bonds. The sequence of the protein in the helical region consists of multiple repeats of the form –Gly–X–Y–, where X is often proline and Y is often a modified proline called 4-hydroxyproline. The glycine residues are located along the central axis of the triple helix, where tight packing of the protein strands can accommodate no other residue. For each –Gly–X–Y– triplet, one hydrogen bond forms between the amide hydrogen atom of glycine in one chain and the carbonyl oxygen atom of residue X in an adjacent chain. Hydrogen bonds involving the hydroxyl group of hydroxyproline may also stabilize the collagen triple helix. Unlike the more common α helix, the collagen helix has no intrachain hydrogen bonds. There is also some covalent crosslinking within the collagen molecule and crosslinking between molecules. In addition to hydroxyproline, collagen contains an additional modified amino acid residue called 5-hydroxylysine. Some hydroxylysine residues are covalently bonded to carbohydrate residues, making collagen a glycoprotein. The role of this glycosylation is not known. The more crosslinking the less soluble to water the collagen is. The smallest amino acid is Glycine and it is this amino acid that resides on the inside of the triple helix structure with its hydrogen atom facing inward. Two more common amino acids are Proline and Hydroxyproline and face outward. This gives the polypeptide chain its characteristic helical shape. [1] [2] [3] [4] [5] If collagen is hydrolyzed, the three amino chains are separated into a random glob, while still being bonded to adjacent chains with a peptide bonds and some hydrogen bonding. This is now the nature of gelatin. Because the structured arrangement has been broken down, the gelatin will have partial triple helices with loose ends bonded to other polypeptide strands and loose polypeptide strands bonded to other loose polypeptide strands forming a matrix of connected fully and partially broken down collagen molecules. It is this Random Coil that give gelatin its springy properties. [6] [7] These two images were taken from source. [8]
Triple helix of collagen (crosslinked to another molecule from peptides at end of molecule)
Collagen molecules line-up to form a fibril in "quarter staggered" array.
Gelatin
Gelatin is made by using the Hydrolysis process to get water to react with the Collagen. The Collagen undergoes partial hydrolysis and is broken down into the Random Coil Globs. The intermolecular and intramolecular bonds that render collagen insoluble to water has to be broken as well as the hydrogen bonds holding the triple helix together has to be broken. The amount of water bonded directly to the gelatin is about 12% - 14% after hydrolysis and after the gelatin is allowed to dry. As the newly formed gelatin cools, hydrogen bonds reform, forming the Random Coil Globs. Gelatin dehydrated to 2% water becomes insoluble in water because of the extensive crosslinking and is achieved by dehydraion. It is this water bonding to the polypeptide chains that keeps the chains from crosslinking. Crosslinking is the covalent (sharing of 1 or more electrons) bonding of the polypeptide chains. This gelatin can be reheated in water to break down the hydrogen bonds again and then redried. It is this latter part that we use to make emulsion. [6] [9] [10] Gel Strength of gelatin is a measure of the rigidity of a gel formed from a 6.67% solution and prepared according to certain arbitrary prescribed conditions. [11] [12] Bloom (named after Mr Bloom whom invented the measuring device) is a measure of force (weight) required to depress a prescribed area of the surface of the sample a distance of 4 mm. The more rigid the sample the higher the bloom. [11] [12] This image was taken from source. [8]
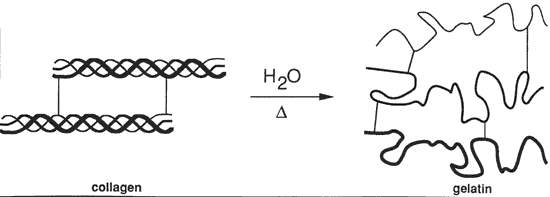
Denaturation of collagen
CrVI
Hexavalent chromium CrVI compounds are a group of chemical substances that contain the metallic element chromium in its positive-6 valence (hexavalent) state and can be found naturally in rocks but is most commonly produced by industrial processes. It has the ability to gain electrons from other elements (a strong oxidizer), which means it can react easily with them. [13] [14] Research is needed using vitamin C with CrVI. [15]
DCG
When Dichromate is added to a gelatin emulsion and then dried the compound is in a clear dissolved up state in a gelled solution. The Chromium is still in the CrVI state. On exposure to the appropriate light source (actinic radiation) the Chromium gains an electron by oxidizing some of the amino acid groups (Where from and how does it gain this electron?) and becomes CrV very quickly and easily. This CrV is bound more tightly then CrVI to the gelatin and cannot be easily washed away with just water. With continued exposure some of the CrV gains more electrons and becomes CrIII but this happens much more slowly then the creation of CrV from CrVI. After exposure the, in the light struck areas, there is a large amount of semi-strong bounded CrV and traces of CrIII causing crosslinking. If this latent hologram is allowed to sit in the dark, the CrV continues to gain electrons (from where?) and converts to CrIII causing additional crosslinking. Because the dark reaction of CrVI to CrV is also slow, more CrIII and more crosslinking in formed in the light struck areas CrV to CrIII, then in the non light struck areas, CrVI to CrV to CrIII. [16] During the first step of processing (reducing agent: Fixer or Sodium Metabisulfite) the CrV is very quickly changed to CrIII and ultimately causes more crosslinking in the light struck areas of the gelatin. The CrVI is washed out as the reducing agent works much more slowly on CrVI to CrV to CrIII. So we have now just increased the crosslinking much more in the light struck areas then in the non light struck areas. And it is this highly crosslinked area of the gelatin that has a higher index of refraction then the uncrosslinked areas yielding us our phase hologram. [16] The DCG hologram is then washed to remove all traces of the reducing agent, unbound Cr. and any loose gelatin. Remember, gelatin is soluble in water unless it is crosslinked. The water also has the effect of swelling the gelatin and thus the fringes so a hologram is still not visible until the gelatin and fringes have been shrunk back to their original size or at least shrunk to a size able to replay the visible wavelengths. The Hologram is then put into an alcohol bath. Many techniques have yielded good results in varying the temperature, duration, concentration and the number of these alcohol baths with each variable changing the final appearance of the hologram. The goal of the alcohol is to remove the water bound in the gelatin structure without allowing a collapse of the delicate fringe lattice structure. (Does alcohol bond where the water was bonded?) (How does alcohol absorb water?) Once the water has been unbound the hologram can be dried with forced or latent heat thus evaporating the alcohol and more of the now scarce water. Again, the more moisture that is taken out of the emulsion, the more crosslinking there is (even in unexposed regions) and the more insoluble the emulsion is. When taken below 2% water content the emulsion is insoluble at room temperature due to being fully crosslinked.
Footnotes
- ↑ http://en.wikipedia.org/wiki/Collagen
- ↑ http://www.britannica.com/eb/article-72553/protein
- ↑ http://www.lsbu.ac.uk/water/hygel.html
- ↑ http://www.stanford.edu/~spark7/
- ↑ http://sandwalk.blogspot.com/2007/02/collagen.html
- ↑ 6.0 6.1 http://en.wikipedia.org/wiki/Gelatin
- ↑ http://www.lsbu.ac.uk/water/hygel.html
- ↑ 8.0 8.1 http://aic.stanford.edu/sg/bpg/annual/v10/bp10-09.html
- ↑ http://albumen.stanford.edu/library/c20/kozlov1983.html
- ↑ http://www.greatlakesgelatin.com/gelatin%20information.htm
- ↑ 11.0 11.1 http://www.gelatin-gmia.com/PDFs/2.1%20Gel%20Strength.pdf
- ↑ 12.0 12.1 http://www.gelatin-gmia.com/index.htm
- ↑ http://www.cdc.gov/niosh/topics/hexchrom/
- ↑ http://solutions.3m.com/wps/portal/3M/en_US/OH-ESHexChrom/Hexavalent_Chromium/
- ↑ http://en.wikipedia.org/wiki/Hexavalent_chromium
- ↑ 16.0 16.1 Improving the remarkable photosensitivity of dichromated gelatin for hologram recording in green laser light. Jeff Blyth, Christopher R. Lowe, John F. Pecora
Additional References
- Dark self-enhancement in dichromated-gelatin grating: a detailed study. Roma Grzymala and Tuula Keinonen
- http://www.pslc.ws/mactest/gel.htm
- http://www.polymerexpert.biz/PolymersandComposites.html